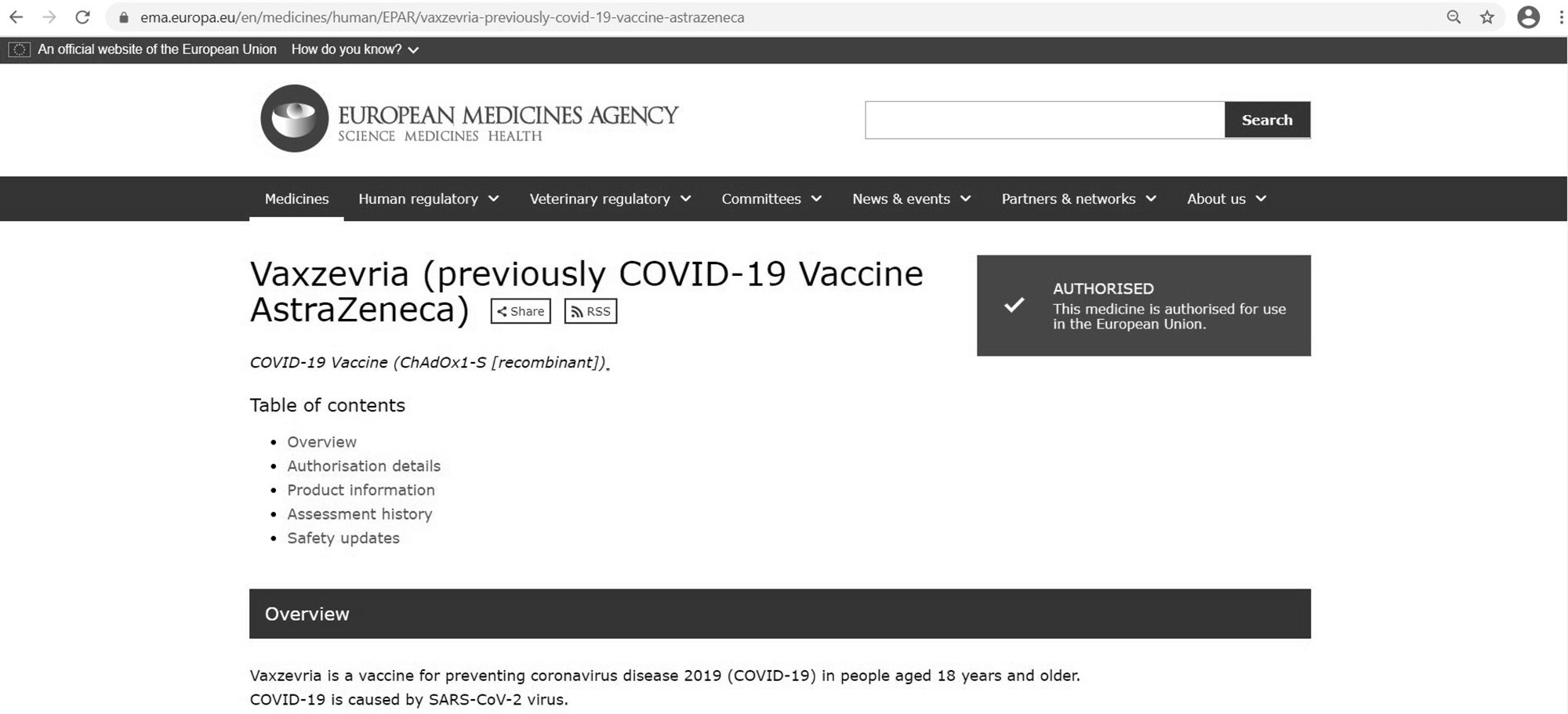
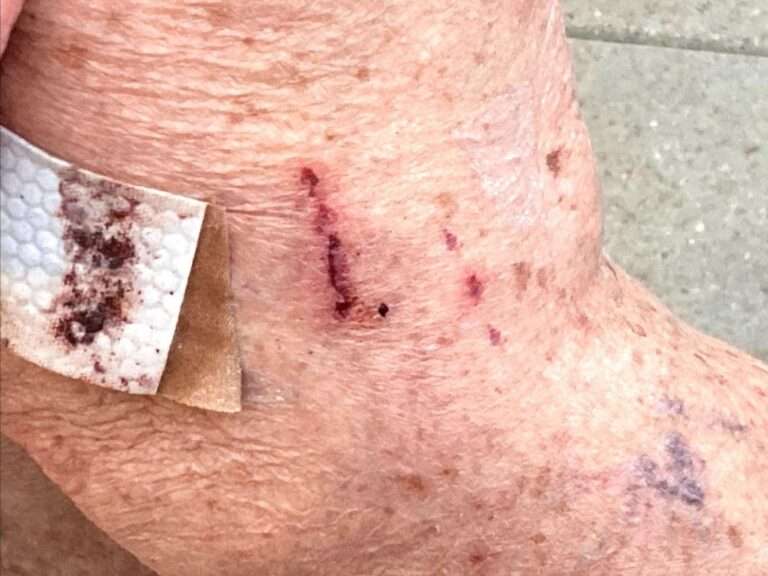
Just days after the German government banned the use of the AstraZeneca COVID-19 vaccine on people under the age of 60 after a series of thrombosis related deaths the vaccine has been renamed ‘Vaxzevria’.
The British-Swedish pharmaceutical company headquartered in the city of Cambridge in England said the drug would be sold on the European market under the new name ‘Vaxzevria’ but the vaccine itself would not undergo any changes.
The name change was approved by the European Medicines Agency (EMA) on 25th March.
The EMA has changed the name of the vaccine on its official website which now reads ‘Vaxzevria’ (previously COVID-19 Vaccine AstraZeneca)’.
AstraZeneca said in a press release that medical workers should be made aware of the rebrand as the packaging of the drug may change, however, the statement did not clarify why the name change was made.
This is not the first time the vaccine has undergone a name change as it was initially sold as ‘AZD1222’ before it was changed to ‘COVID-19 Vaccine AstraZeneca’.
According to the German news outlet TAG 24, the name change is not an uncommon practice as the vaccine is already manufactured and sold in India under the name ‘Covishield’.
The vaccine which was approved by the EMA in late January 2021 has been under pressure recently due to reports of patients developing Thrombosis after receiving it.
The timing of the name change comes just days after Germany banned the use of the vaccine on people under the age of 60 because of thrombosis risk.
Canada announced on Monday (29th March) that it was suspending the use of the vaccine on patients under the age of 55.
The EMA confirmed on 18 March that the vaccine was safe and effective and that the benefits outweigh the possible risks, but is still investigating whether the thrombosis cases are correlated to the newly renamed vaccine.
Despite the EMA’s approval, a poll released by YouGov last Monday (29th March) revealed that only 23 percent of French people and 32 percent of Germans consider the vaccine to be safe.
AstraZeneca has not confirmed whether or not the name change was driven by an attempt to counter the current bad publicity surrounding the vaccine.
To find out more about the author, editor or agency that supplied this story – please click below.
Story By: Georgina Jadikovska, Sub-Editor: Joseph Golder, Agency: Newsflash
The Ananova page is created by and dedicated to professional, independent freelance journalists. It is a place for us to showcase our work. When our news is sold to our media partners, we will include the link here.